Date
Health and life sciences
Staying ahead: the case for a European Action Plan for Rare Diseases
Share
This report and its accompanying event, “Rare Diseases Forum 2024: Securing Europe’s competitiveness in R&D for people living with rare diseases”, has been supported by a consortium of partners who all share a common goal to advocate for people living with rare diseases.
Foreword
In September 2023 at the UN Headquarters, ministers, advocates and even royal representatives from all corners of the globe declared their commitment to universal health coverage for all people living with rare diseases. While the world has seen a great deal of progress on rare diseases, our global community needs to reinforce this momentum. 2024 marks a vital year: an unprecedented proportion of the global population, including European citizens, will be voting in elections. Now is the time to inform and shape the future actions of policymakers on rare diseases through the strength and clarity of our collective voice.
For European policymakers, a key priority is “competitiveness”, making sure Europe can stay ahead in a global race to secure a strong, resilient economy with prosperous, healthy societies. Europe’s competitiveness on the world stage truly matters for people living with rare diseases. The consequences of falling behind could potentially be critical. Therefore, we need to continuously invest in basic science and R&D conducted in European countries, supporting companies to scale and develop products, and ensure that access to novel therapies is truly equitable across the continent, with significant knock-on benefits for families and caregivers, not to mention the economy of the wellbeing.
Innovative therapies are not developed in a vacuum, even with the best regulatory framework. The the European research-based biopharmaceutical companies need an ecosystem of infrastructures, policies and collaborations which are conducive to knowledge generation and innovative scientific, medical, regulatory, healthcare approaches. There is growing momentum for a comprehensive European Action Plan for Rare Diseases, to leverage robust EU policy initiatives towards concrete objectives that will help enhance the competitiveness of the health sector for the benefits of people living with a rare disease. As many European nations are also developing their own national strategies to tackle rare diseases, we must ensure that this political trajectory towards collective action is strengthened.
As the European Union prepares to welcome the new European Commission and MEPs for the 2024-2029 term, EURORDIS and Europe’s broader rare disease community stand ready to collaborate with policymakers. Such collaboration is key to achieving significant progress in enhancing the lives of those living with rare diseases, and addressing their substantial unmet needs through effective and concrete policy actions.
Yann Le Cam
Outgoing Chief Executive Officer
EURORDIS
Executive Summary
The challenges faced by the over 30m people living with rare diseases in Europe are significant. The relative cost burden for patients, families, and caregivers is far higher for rare diseases compared to common illnesses. Rare disease patients typically wait much longer for a confirmed diagnosis, compounded by the complexity of their condition. As the majority of rare diseases lack an authorised treatment, patients often lack access to curative therapies.
To address these areas of unmet need, the EU has introduced a series of regulations, policies and initiatives over previous decades. Chief among them was the 2000 EU Regulation on Orphan Medicinal Products, which had a positive effect on increasing research and development (R&D) efforts, new drug approvals and patient access to new therapies. Alongside regulations and policies, the creation of European Reference Networks (ERN) provided the necessary coordination of expertise and resources to support patients with rare diseases, where the same approach taken for more common illnesses is insufficient.
Despite these strides forward, Europe is struggling to compete effectively at a global level. In broad terms, such as in R&D expenditure, and more specifically – including in the number of rare disease clinical trials conducted in European countries – Europe is falling behind. In particular, there has been a noticeable decline in the number of orphan drug designations and approvals in the EU in recent years. As the EU’s competitiveness decreases, this risks further reductions in rare disease R&D, innovation, investment and patient access to new treatments.
The European Commission is alive to these trends. The Antwerp Declaration, the Annual Report on the Single Market and Competitiveness, the anticipated work by Mario Draghi and Enrico Letta, and commitments to the European Health Union all demonstrate the level of political ambition to promote a globally competitive biopharmaceutical sector in Europe. In being at the forefront of life sciences innovation – pushing boundaries in clinical trial designs, real-world data, access and uptake of new medicines – the rare diseases sector is well placed to benefit from, and contribute to, Europe’s broader competitiveness agenda.
To seize the opportunity of competitiveness and harness the inherent strengths of Europe’s rare diseases sector, a European Action Plan for Rare Diseases is needed. An Action Plan will provide a comprehensive EU-level policy framework to drive a common strategy across member states. There is considerable political support for an Action Plan; a concept endorsed by 21 member states during Czechia’s Presidency of the Council of the EU and has continued to gather significant momentum. Now is the time to establish an Action Plan which prioritises the over 30m people living with rare diseases, shows that policymakers have listened, and enables one of Europe’s most innovative sectors to stay ahead.
Introduction
Against the backdrop of the Belgian Presidency of the Council of the EU, this report aims to renew and strengthen calls for rare diseases to be a political priority. Through policies, regulations and scientific advancements, much has been achieved over the last 30 years to support Europeans living with rare diseases, and to position Europe as a global leader in rare disease R&D and care. However, Europe is facing increased competition from other developed markets namely the United States (US) and China. By recognising this fundamental challenge, the Commission is prioritising efforts to drive the EU’s long-term competitiveness.
To ensure Europe stays ahead and remains a global leader in rare disease R&D and care, an up-to-date Action Plan is needed. This framework will help in setting member states coordinate on shared goals, amplify those National Action Plans already in place or under development, and support efforts to improve the lives of millions of people impacted by these rare conditions. Furthermore, an Action Plan will support the delivery of broader EU priorities, including Europe’s Beating Cancer Plan and the European Health Union whilst bolstering the competitiveness of the EU Single Market.
Challenges for people impacted by rare diseases
In the EU, a rare disease is defined as one impacting less than five in every 10,000 people. While the number of people impacted by a specific rare disease is small, the total number of people impacted by the ~7000 known rare diseases is much larger: up to 36m people in Europe, around 6% of the population. Not only do rare diseases greatly impact the lives of people suffering from them, their families and caregivers, it also impacts their livelihood and ability to contribute towards the economy.
In European countries, the cost burden on families affected by a rare disease is significant. A 2023 study of citizens in Germany, France and Italy found the average burden for common illnesses (including diabetes, cardiovascular diseases, Alzheimer’s disease, arthritis and certain cancers) to be around €7,000 per patient per year (PPPY), whereas for rare diseases, the average burden is around €107,000 PPPY – 15 times greater. Of this PPPY burden, the indirect costs of rare diseases (e.g., caregiver burden, home changes and costs of secondary treatments, travelling and accommodation) averaged 29% of the total burden when treatment is available, but rose to an average of 45% when no treatment is available. Most of these indirect costs are borne by families.
People with rare diseases also face significant hurdles when seeking an accurate diagnosis and treatment. It takes on average five years for rare disease patients to receive a diagnosis, with 70% waiting over a year for a confirmed diagnosis after presenting to a medical practitioner, and many facing a diagnostic delay of up to 30 years. This suggests that the majority of the time delay between the presentation of symptoms to diagnosis is caused by health system-related processes and inefficiencies.
Once diagnosed, strikingly, 95% of known rare diseases have no dedicated treatment, and those authorised treatments that do exist tend to target a patient’s symptoms, rather than the natural history of the disease. With a severe lack of effective treatments available for the vast majority of rare diseases, there is a strong case for greater investment in discovery research into new therapeutics. European leadership in rare diseases R&D is paramount for its citizens, allowing earlier and better care. Going further, it can also support the EU’s competitiveness agenda and delivery of other key European Commission goals.
Strides forward to tackle rare diseases
Over the past three decades, Europe has taken tremendous strides forward to address the challenges posed by rare diseases. Through policy development, forward-thinking regulations, and collaborative initiatives, there has been sustained investment in research, ground-breaking innovations discovered, and earlier access for patients to novel therapies. From the EU Regulation on Orphan Medicinal Products (No 847/2000) (OMP Regulation), EU Regulation on Advanced Therapy Medicinal Products (No 1394/2007), and National Action Plans at member state-level, to the establishment of European Reference Networks (ERNs) and the Joint Programme on Rare Diseases, numerous beneficial measures have been put in place. There has also been impactful research funding delivered under Framework Programmes and Horizon research, and a willingness to enter public-private partnerships as seen through the Innovative Medicines/Health Initiatives (see Fig 1).
Taken together, these strides have undoubtedly contributed to increases in rare disease R&D, and earlier and better access to new diagnostics and treatments. From 2000-2020, the number of rare disease clinical trials conducted in Europe grew by 88%, and the number of medicines approved to treat rare diseases increased from eight to 167. Over a similar period, it was reported that over half of the OMPs authorised in Europe would not have been economically viable without the OMP Regulation. The impact on patients has been significant. A 2024 study found that, of all OMPs approved between 2010-2021, more than 50% were intended to treat diseases with a prevalence lower than or equal to one in 10,000. The European Commission concluded that the OMP Regulation has added between 210,000-440,000 Quality Adjusted Life Years (QALYs) to the lives of patients in the EU. Moreover, ERNs have proved transformative in supporting patients and families, providing specialist knowledge and resources in the diagnosis and treatment of rare diseases, enabling the benefits of the OMP regulation to be realised.
In 2023, the European Commission proposed revisions to the EU’s General Pharmaceutical Legislation regulating medicines for human use, including proposals to update the EU’s Regulations on Orphan Medicinal Products and Paediatric Medicines. Many patients and families welcome continuous and updated measures that would lead to more rapid regulatory pathways for new products and more targeted incentives for companies to develop more orphan medicines. Whilst the revision is still ongoing, it presents an opportunity to place Europe on a more globally competitive footing with the US and China, particularly in increasing the research, development and accessibility of rare disease medicines. Similarly, the 2021 Health Technology Assessment (HTA) Regulation offers hope of improving access to novel cell and gene therapies. By streamlining the assessment process, encouraging collaboration between companies, launching the European Medicines Agency (EMA) and HTA coordinating group, and defining required evidence at pre- and post-drug launch, the 2021 HTA Regulation provides a further opportunity to improve patient access while also boosting Europe’s competitiveness in developing and approving orphan drugs.
Fig 1. Timeline of rare disease policies and health system initiatives
Warning signs: Europe falling behind the global competition
Despite a favourable regulatory and incentive framework for rare disease R&D and registrations of new orphan drugs, Europe is losing ground to its global competitors. In comparators such as R&D expenditure, private investment and clinical research and data, Europe lags behind the larger markets of the US and China. Without a renewed focus on rare disease research, development and care delivery, this declining trend will likely continue. If Europe fails to stay ahead, the consequences could be significant. Companies could choose to conduct trials in other, more favourable markets, invest in research and new facilities elsewhere, and launch new products in competitor markets first. As a result, Europeans would lack earlier access to novel therapies, and countries would be at risk of gradually losing company sites, investments, technology, skilled workers, intellectual property (IP) and tax revenue, as corporates and investors perceive Europe as a less attractive destination to do business.
Fig 2. R&I expenditure as a percentage of GDP across the US, China, and EU countries (2017-2021)
Research and innovation expenditure
Europe has often led the way in life sciences innovation. However, the sector has not been immune to declines in its performance and faces an uncertain future as competition from the US and China intensifies. US and Chinese pharmaceutical R&I expenditure now outweighs Europe, as do R&I employment figures. While the EU’s R&I investments have increased from 1.8% of GDP to 2.2% in 20 years, they remain below the target of 3%, and below those in the US (3.4% of GDP) and China (2.4%) (Fig. 2). Comparing the share of global pharmaceutical R&D spend across the US, China and Europe in 2020, Europe contributed 32%, but this is projected to fall to 21% by 2040. In China, since 2010, employment in pharmaceutical R&D has dramatically risen to over 134,000. This is just under the US at 138,000, with Europe in third at 121,000. While employment in pharmaceutical R&D in Europe has grown, this has not made up for larger gains in the US and China.
Fig 3. Venture capital investment in rare disease R&D (2018-2022)
Private investment in rare diseases R&D
Venture capital (VC) investment is often a barometer for the global attractiveness of a sector and willingness of investors to back its new and emerging companies. Like the biotech sector overall, the US dominates funding in rare disease R&D. For example, from 2018-2022, the US led the way with close to 45,000 VC deals in rare disease drug R&D, totalling $1.01bn. APAC’s VC market was second with deal values at $363m and Europe in third at $307m. Median VC deal sizes in Europe (2016-2022) were around $10m, although this rose to around $45m in 2023. In comparison, the US, with a more mature VC market, demonstrated a median deal size of $60m (2016-2022), rising to $150m in 2023 (Fig. 3). In Europe, where the biotech VC ecosystem is smaller, less mature, and more fragmented than other more mature ecosystems, such as in Boston and San Francisco in the US, are increasingly directed towards late-stage programmes.
Fig 4. Percentage of clinical trials for rare diseases by geography
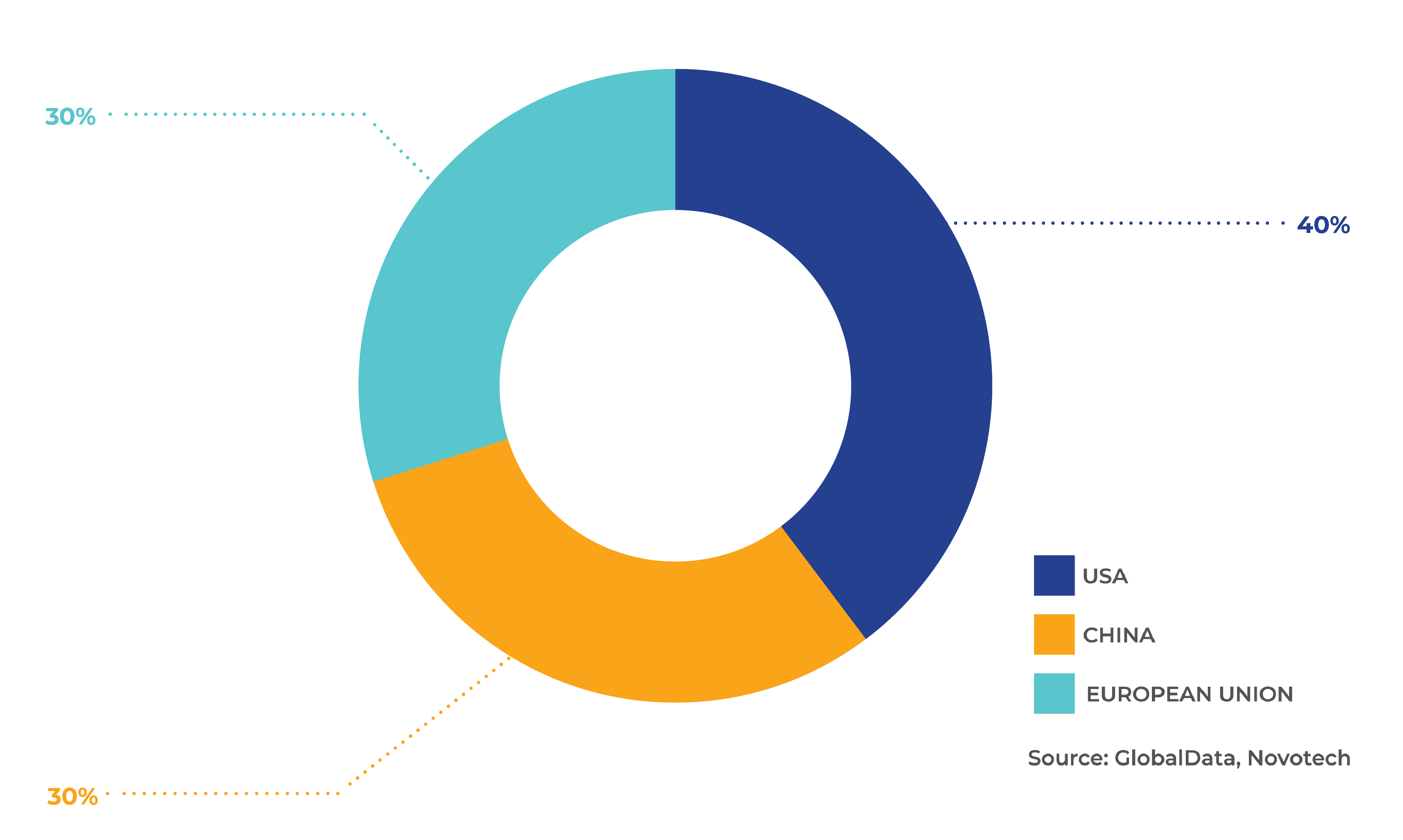
Clinical research and data
Between 2018-2022, there were 16,000 rare disease clinical trials globally. By region, APAC accounted for 38%, North America 30%, Europe 23% and Rest of World (RoW) 9%. Across the US, China, and Europe specifically there were 11,844 trials, of which the US made up 40%, China 30% and Europe 30% (Fig. 4). In this period, the number of rare disease clinical trials in all regions grew, except in Europe. In terms of trial size from 2018, it was found that larger scale trials were conducted in the US, with an average size of 456 people, followed by the Europe (244) and Japan (67). The 2020 report by EurActiv and EUCOPE found there had been a decline in the number of designations and approvals in the Europe in the past four years. Key hurdles were access to data and the collection of real-world evidence. It was acknowledged that Europe’s competitiveness was decreasing, and this risked further reductions in innovation. As proposed in the Rare 2030 Foresight study, data on people living with rare diseases needs to be optimised and fully utilised for patient and societal benefit.
Investment in rare disease R&D, driven by well-informed policies and regulatory frameworks, can contribute towards Europe’s global competitiveness in life sciences overall. Owing to the inherent challenges of conducting research into rare diseases, it is an area that often breaks new scientific ground, including through new clinical trial designs and unique ways of generating Real-World Data. Through collaboration with regulators and payers, Europeans can be among the first to benefit from novel therapies. Investment in Europe’s R&D also brings indirect benefits in other areas of the economy and value chain, attracting talent and skills and ensuring that high value add products are developed and produced in Europe. Overall, rare diseases R&D and care is an exemplar of innovation within the life sciences sector, and an area where Europe can demonstrate its added value on the world stage.
Securing Europe’s competitiveness in rare disease R&D and care
The pharmaceutical sector is integral to European competitiveness. In 2019, medicine producers made the biggest contribution to European research investment with over €37bn, providing 800,000 direct jobs and a €109.4bn trade surplus. In 2023, of all sectors, the European pharmaceutical sector had the largest trade surplus. Emerging biopharmaceutical companies account for over 70% of the research pipeline, contributing to a vibrant sector. Taking into account previous rare disease policies and regulations, and areas where Europe has started to fall behind, the European Commission’s renewed focus on competitiveness offers a fresh opportunity to explore how Europe can level the playing field.
The launch of the Antwerp Declaration by President von der Leyen and Prime Minister De Croo further sharpened the European Commission’s focus on global competitiveness and resilience, particularly in growth sectors seeing strong investment in China and the US, including the biopharmaceutical sector. Through the European Commission’s recent Annual Single Market and Competitiveness Report and commitments towards developing the European Health Union and other Communications, the following areas could offer opportunities to contribute to, and benefit from, the rare diseases sector.
Leveraging the Single Market
A well-functioning Single Market and industrial strategy is critical to ensuring that Europe continues to compete. The importance of European competitiveness will be further underlined by the work of Mario Draghi and Enrico Letta in their respective reports on the future of European competitiveness and the Single Market, which are expected to call for strengthened support for innovative technologies across key sectors, including pharmaceuticals. In turn, a competitive rare disease sector, supported by an accessible Single Market, has the potential to drive innovation across broader disease areas, clinical trial methodologies, and approaches to access and reimbursement.
Building the European Health Union
More broadly, the Commission has taken positive steps towards developing the European Health Union. The announced €50bn investment by 2027 - to bring innovation, scientific capacity, private sector knowledge and competent national authorities together – was widely welcomed. Whilst the aims of the European Health Union focused on pandemic preparedness, Europe’s life sciences sector and rare disease research and care will likely benefit. As part of this, European countries are expected to prepare and respond together to health crises, ensure medical supplies are available, affordable and innovative, and work together to improve prevention, treatment and aftercare. However, a European Health Union will not be enough to address the significant unmet needs faced by people living with rare diseases, for whom research and treatments require targeted funding initiatives, detailed policy frameworks and productive collaborations between all stakeholders.
Supporting Research, Development and Innovation across Europe
Research & Innovation is one of nine key drivers of the EU’s 2023 Long Term Competitiveness Communication, and is directly boosted by a focus on rare diseases. Rare disease R&D is not only at the cutting edge of diagnostic and drug development – it also drives our understanding and ability to effectively treat more common illnesses. Clinical research into rare diseases involves small, highly dispersed patient populations, requiring the development of innovative approaches to clinical trial design that can then be used to improve trials in other disease areas. The same is true of commercial agreements and access arrangements. Rare disease R&D has the potential to drive innovation across the pharmaceutical value chain. Support for rare disease R&D directly contributes to the delivery of the Pharmaceutical Strategy for Europe, which prioritises unmet medical needs and rare diseases, recognizing that treatments in many disease areas are still lacking. The Strategy is, in turn, a key pillar of the European Health Union.
Boosting Capital Investment
In its Single Market and Competitiveness Report, the Commission identifies the need to increase the availability of risk and venture capital funding to support the scale up of innovative companies. The European biotech ecosystem for rare diseases is well-positioned to be a global leader, but more policy support is needed at national and EU-level to nurture startups and create sustainable pathways for company growth in the region, including through partnership and collaboration with more mature partners. Policymakers are waking up to the need to boost business incentives and increase access to scale-up financing by tapping into deeper pools of institutional investor capital, as seen in France through the “Tibi Initiative”. EU-level incentives, such as the orphan designation, are also critical to create a level-playing field across regions and enable biotech companies to attract the necessary capital for preclinical and clinical development. A key ingredient to global competitiveness is supporting self-sustaining business ecosystems that create virtuous circles of innovation, investment and skilled jobs, as seen in Boston and San Francisco in the US.
The Call for a European Action Plan for Rare Diseases
There is a golden opportunity to transform patients’ lives, support one of Europe’s prized sectors, and strengthen its global standing. In the context of Europe’s competitiveness agenda, and building on all previous efforts, the case for a European Action Plan for Rare Diseases has never been stronger – a Plan that prioritises the unmet needs of patients and by doing so, stimulates a more innovative and competitive rare disease sector.
Why an Action Plan is needed
As Europe faces up to the challenges of addressing unmet needs and remaining globally competitive, a European Action Plan for Rare Diseases is now required to:
Address remaining unmet needs and inequities all along the patient journey in accessing a diagnosis, treatments and care, and preventing those living with a rare diseases from being marginalised in society.
Keep pace with new technologies, new values and new expectations of Europe’s citizens, giving a new focus to national rare disease plans and strategies that incorporates Europe’s broader ambitions to remain globally competitive.
Sustain the European Commission’s strategic approach in addressing a distinctive domain of high European added-value and bring together existing and upcoming actions, across countries, across sectors and policy areas, and across the rare disease pathway, where Europe can add the most value under one interconnected framework.
Modernise EU-level strategies that have become outdated. Whilst great strides have been made, particularly through the 2008 Commission Communication and Council Recommendation on Rare Diseases, these are now outdated and fail to account for our greater understanding of diseases and how to treat them effectively.
How we achieve it
A core principle of this Call to Action is building on all the previous efforts that have helped the sector reach this point, and supporting those efforts still ongoing and those yet to begin. Specifically, achieving an Action Plan will likely involve:
Building on the foundations laid by EURORDIS and the wider rare diseases ecosystem. The Rare 2030 Recommendations, and subsequent Working Proposal provide a strong basis for a comprehensive policy framework, developed after a two-year study with over 250 experts from across the rare disease community. Whilst political, economic and scientific landscapes may have shifted since publishing in 2021, this work provides a blueprint to inform a future Action Plan.
Leveraging significant political momentum at EU level. Twenty-one EU member states endorsed a call to action under the Czech Presidency of the Council of the EU in 2022. A European Action Plan was also promoted under both the French and Spanish Presidencies of the Council in 2022 and 2023 respectively. Since 2009, the European Economic and Social Committee (EESC) has been supporting a comprehensive EU-level approach that takes into account all the needs of people living with rare diseases. On Rare Diseases Day 2024, the European Parliament’s Subcommittee on Public Health (SANT) heard strong voices of support from MEPs for urgent EU-wide action on rare diseases. The European Parliament in its Resolution on the EU public health strategy in the post COVID-19 era also called for a European Action Plan on rare and neglected diseases. Moreover, all but two member states have either established or are currently developing, National Action Plans for rare diseases to ensure they remain a public health priority.
Supporting new initiatives with a shared goal. An Action Plan must complement new initiatives and Joint Actions being launched by the Commission. For example, the new €18m Joint Action (JARDIN) aims to improve the diagnosis, treatment, and care of patients with rare diseases by integrating ERNs into national health systems. This brings together all member states, as well as Norway and Ukraine, and will address issues such as patient pathways and data management for rare diseases. In doing so, JARDIN will pave the way for even more effective, efficient, and sustainable cooperation in the future.
Aligning with the United Nation’s Sustainable Development Goals (SDGs). As advocated by EURORDIS, a European Action Plan would also be aligned to the UN’s SDGs– particularly goals 3 (good health and wellbeing), 5 (reducing inequalities) and 9 (industry, innovation and infrastructure) – and would achieve this through genuine partnerships with governments, the private sector and civil society.
Looking ahead to subsequent Council Presidencies. An Action Plan will only be achieved through sustained advocacy at both EU and national level. member states assuming the Council Presidency over the coming years should ready themselves to pick up Belgium’s torch which has shone new light on the case for a European Action Plan and requires other nations to do the same.
Conclusion
Many policies and initiatives have been designed and implemented in Europe over the last 30 years to support rare disease R&D. Nonetheless, there is a need to build on previous successes and go further. It is clear that Europe’s current approach to rare diseases – which lacks an overarching, up-to-date strategic framework at EU-level with strong political will behind it – will not be able to compete internationally. The consequences of this could be severe: declines in business investment, skilled jobs, tax revenue, access to clinical trials, drug launches, and equal access to medicines. In turn, the economic burden on people living with rare diseases, their families and carers, and the wider economy would be equally significant.
An Action Plan for Rare Diseases will enable Europe to stay at the forefront of rare disease R&D and treatments, and in doing so, stimulate spill-over innovations in the wider life sciences sector, supporting patients and Europe’s global standing. Despite numerous calls by member states, Europe still lacks a comprehensive strategy on rare diseases that integrates all the different areas of the sector – from basic science and clinical research, to Real World Data and Evidence, to healthcare delivery - that also incorporates all member states in a common strategy at the European level. With the Czech Presidency’s proposal for an Action plan receiving wide approval from member states – and 23 national strategies for rare diseases, either adopted or being developed – there is already strong “bottom-up” development. This goes alongside support from the European Parliament and EESC. A European Action Plan would constitute a “top-down” approach that affirms policymaker commitments to support people living with rare diseases.
The views expressed in this report can be attributed to the named author(s) only.
